Bioactivity Supports Premature Infants’ Immunity, Development, and Growth, According to a Literature Review
DUARTE, Calif. (June 12, 2023) – Prolacta Bioscience, the world’s leading hospital provider of 100% human milk-based nutritional products for critically ill, premature infants, today announced that Breastfeeding Medicine has published an independent study demonstrating Prolacta’s human milk-based fortifiers (HMBF) reinstate and significantly boost bioactive proteins when added to mother’s own milk (MOM) and donor human milk (DHM).1 The naturally occurring bioactive components in human milk are thought to support infants’ immunity, development, growth, and long-term health.2
The study, “Exclusive Human Milk Diet for Extremely Premature Infants: A Novel Fortification Strategy That Enhances the Bioactive Properties of Fresh, Frozen, and Pasteurized Milk Specimens” was an observational feasibility study that analyzed the bioactive components of human milk to evaluate fortification choices. It was authored by Professor Roy K. Philip, MD, MBA, and colleagues of the University of Limerick School of Medicine, and Bernal Institute, University of Limerick, Ireland.
“Freshly expressed mother’s milk fortified with HMBF appears to be an optimal nutritional choice for extremely premature infants during the critical early postnatal window of tissue accretion and immunological imprinting,” Dr. Philip said. “Pasteurized donor human milk showed significantly low bioactive properties in comparison to fresh and frozen MOM; however, the addition of HMBF reinstated and further enhanced bioactivity to partially offset the effects of pasteurization.”1
Promising Bioactivity Results
- The study found milk samples fortified with Prolacta’s HMBF contained higher protein, fat, and total solids (p < 0.05) compared to unfortified samples and those fortified with cow milk-based fortifier (CMBF). HMBF reinstated lactoferrin and α-lactalbumin and exhibited higher protein, fat, and total solids (p < 0.05) in comparison to unfortified specimens and those supplemented with CMBF.1
- Samples with added HMBF had the highest (p < 0.05) antioxidant activity (AA), suggesting the potential capability of HMBF to enhance oxidative scavenging.1
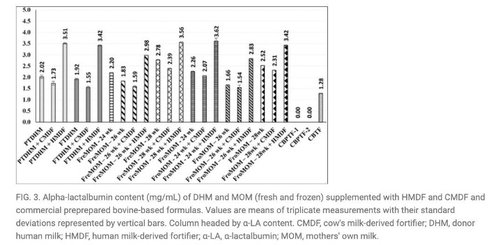
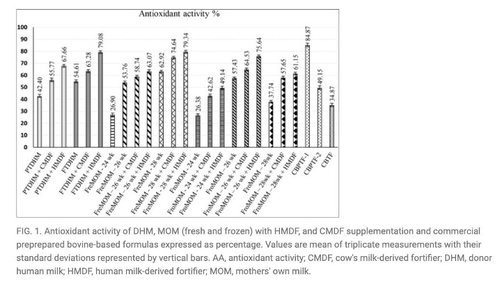
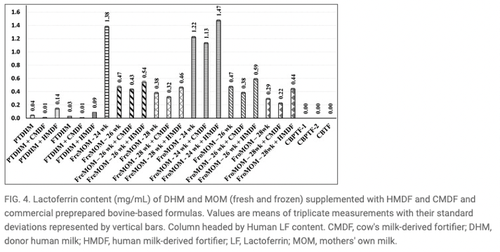
Source for Figures 1, 3 and 4: Philip RK, Romeih E, Bailie E, et al. Exclusive Human Milk Diet for Extremely Premature Infants: A Novel Fortification Strategy That Enhances the Bioactive Properties of Fresh, Frozen, and Pasteurized Milk Specimens, Breastfeeding Medicine. 2023;18(4) doi: 10.1089/bfm.2022.0254
“This study highlights the advantages of Prolacta’s proprietary processing in retaining important bioactive components," said Melinda Elliott, MD, chief medical officer at Prolacta. “The findings underscore that bioactivity matters and may be a key reason why extremely premature infants fed an Exclusive Human Milk Diet (EHMD) with human milk-based fortifiers fare better, have fewer complications, and go home sooner than those fed a cow milk-based diet.”3,4,5,6,7,8,9,10,11,12,13,14,15,16
On June 27, Professor Philip will present his study findings in a webinar titled Exclusive Human Milk Diet (EHMD) for Extremely Premature Infants: a Fortification Strategy to Enhance Bioactivity. The registration link can be found here.
About Human Milk-Based Products
Human milk-based products differ from cow milk-based products primarily in their composition — notably, the bioactive components that are exclusive to human milk and thought to support infants' immunity, development, growth, and long-term health.2 These bioactive components, such as immunoglobulins, lactoferrin, milk fat globule membrane, and the wide spectrum of prebiotics known as human milk oligosaccharides (HMOs), are not easily manufactured, and thus are greatly decreased or missing from cow milk-based products.17 Prolacta's 100% human milk-based nutritional products offer the highest bioactivity in the human milk industry due to vat pasteurization, which retains more bioactivity compared to other processing methods like retort sterilization or ultrahigh-temperature (UHT) processing, as supported by multiple studies.16,18,19 Prolacta’s human milk-based fortifiers (HMBF) are clinically proven to reinstate and significantly boost bioactive proteins when added to mother’s own milk (MOM) and/or donor human milk (DHM).1
About Prolacta Bioscience
Prolacta Bioscience® Inc. is a privately held, global life sciences company dedicated to Advancing the Science of Human Milk® to improve the health of critically ill, premature infants. Prolacta’s 100% human milk-based nutritional products have been evaluated in more than 20 clinical studies published in peer-reviewed journals. More than 90,000 premature infants have benefited from Prolacta’s nutritional products worldwide to date.20 Established in 1999, Prolacta is the world’s leading provider of human milk-based nutritional products for hospital use and is also exploring the therapeutic potential of human milk across a wide spectrum of diseases. Prolacta maintains the industry’s strictest quality and safety standards for screening, testing, and processing donor human milk. Operating the world’s first pharmaceutical-grade human milk processing facilities, Prolacta uses vat pasteurization and a patented, FDA-reviewed manufacturing process to ensure pathogen inactivation while protecting the nutritional composition and bioactivity of its human milk-based products. Prolacta is a global company with headquarters in Duarte, California, and can be found online at Twitter, Instagram, Facebook, and LinkedIn.
# # #
Media Contact:
Loren Kosmont
Lkosmont@prolacta.com
310-721-9444
References
- Philip RK, Romeih E, Bailie E, et al. Exclusive Human Milk Diet for extremely premature infants: A novel fortification strategy that enhances the bioactive properties of fresh, frozen, and pasteurized milk specimens. Breastfeeding Medicine. 2023;18(4):279-290. doi:10.1089/bfm.2022.0254
- Gila-Diaz A, Arribas SM, Algara A, Martín-Cabrejas MA, López de Pablo ÁL, Sáenz de Pipaón M, RamiroCortijo D. A review of bioactive factors in human breastmilk: a focus on prematurity. Nutrients. 2019;11(6):1307. doi:10.3390/nu11061307
- Bergner EM, Shypailo R, Visuthranukul C, et al. Growth, body composition, and neurodevelopmental outcomes at 2 years among preterm infants fed an exclusive human milk diet in the neonatal intensive care unit: a pilot study. Breastfeed Med. 2020. 15(5):304-311. doi:10.1089/bfm.2019.0210
- Huston R, Lee M, Rider E, et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk-based fortifier. J Neonatal Perinatal Med. 2020;13(2):215-221. doi:10.3233/NPM-190300
- Rahman A, Kase J, Murray Y, et al. Neurodevelopmental outcome of extremely low birth weight infants fed an exclusive human milk diet is not affected by growth velocity. Breastfeed Med. 2020;15(6):362369. doi:10.1089/bfm.2019.0214
- Lucas A, Boscardin J, Abrams SA. Preterm infants fed cow's milk-derived fortifier had adverse outcomes despite a base diet of only mother's own milk. Breastfeed Med. 2020;15(5):297-303. doi:10.1089/bfm.2019.013
- Delaney Manthe E, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19(6):460-467. doi:10.1097/ANC.0000000000000676
- O'Connor DL, Kiss A, Tomlinson C, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial [published corrections appear in Am J Clin Nutr. 2019;110(2):529 and Am J Clin Nutr. 2020;111(5):1112] Am J Clin Nutr. 2018;108(1):108-116. doi:10.1093/ajcn/nqy067
- Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet [published correction appears in Breastfeed Med. 2017;12(10):663] Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134
- Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216-220. doi:10.1038/jp.2015.168
- Abrams SA, et al. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med. June 2014. 9(6): 281-0285. doi:10.1089/bfm.2014.0024 This cohort study included 260 extremely preterm infants born weighing less than 1,250 g who received a diet that ranged from 100% cow milk to 100% human milk.
- Hair AB, Hawthorne KM, Chetta KE, et al. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res Notes 2013;6:459. doi:10.1186/1756-0500-6-459.
- Cristofalo EA, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. December 2013. 163(6):1592-1595. doi: 10.1016/j.jpeds.2013.07.011.
- Ganapathy V, et al. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. February 2012. 7(1):29-37. doi: 10.1089/bfm.2011.0002. This cost-effectiveness analysis of 2,560 extremely premature infants less than 28 weeks gestational age in 257 hospitals nationwide compared the impact of an Exclusive Human Milk Diet composed of mother’s milk fortified with a human milk-based fortifier versus mother’s milk fortified with cow milk-based fortifier.
- Sullivan S, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. April 2010. 156(4):562-567. doi: 10.1016/j.jpeds.2009.10.040.
- Liang N, Koh J, Kim BJ, et al. Structural and functional changes of bioactive proteins in donor human milk treated by vat-pasteurization, retort sterilization, ultra-high-temperature sterilization, freeze-thawing and homogenization. Front Nutr. Published online September 15, 2022. doi:10.3389/fnut.2022.926814
- Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013 Feb;60(1):49-74. doi:10.1016/j.pcl.2012.10.002. PMID: 23178060; PMCID: PMC3586783.
- Lima HK, Wagner-Gillespie M, Perrin MT, Fogleman AD. Bacteria and bioactivity in Holder pasteurized and shelf-stable human milk products. Curr Dev Nutr. 2017;1(8):e001438. doi:10.3945/cdn.117.00143
- Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact. 2018;34(1):120-129. doi:10.1177/0890334417710635
- Data on file; estimated number of premature infants fed Prolacta's products from January 2007 to December 2022.


