Tell us how we can help
Clinician-to-clinician guidance
Coding, billing, and reimbursement questions
Finding my sales representative
Support with an order
More than 20 clinical studies with more than 5,000 premature infants have demonstrated the effectiveness of Prolacta’s 100% human milk–based nutritional products.1
Our products, as part of an EHMD, have been shown in clinical studies to:
Prolacta’s human milk–based nutritional products contain a wide range of human milk oligosaccharides (HMOs)—special sugars abundantly found in human milk.9 HMOs promote the development and maturation of the newborn immune system and provide a supplementary source of sialic acid critical for brain development. 10,11
Prolact+ H2MF is the first commercially available human milk fortifier made from 100% human milk.

Human milk caloric fortifier is ideal for neonatal infants receiving low caloric content. Data show that 65% of the time, term mother’s own milk (MOM) is less than 20 Cal/fl oz.12 Prolact CR human milk caloric fortifier can meet the need for additional calories.

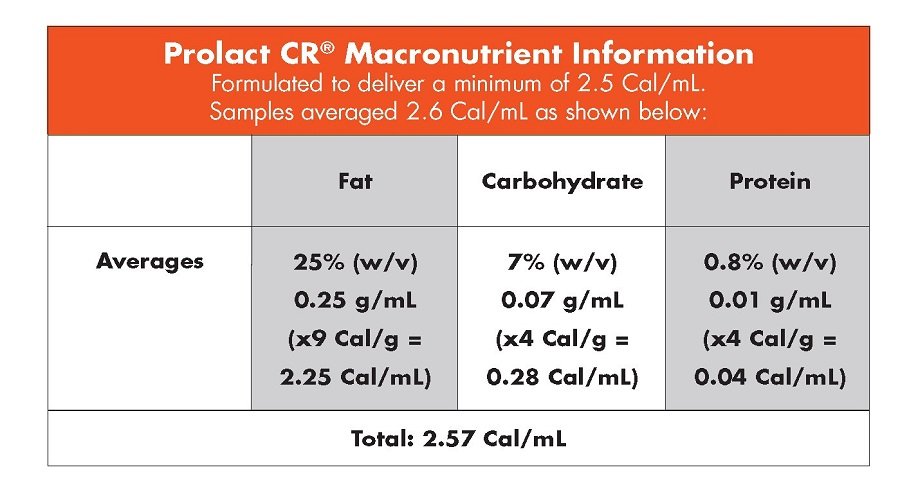
Prolact CR human milk caloric fortifier is the only completely human solution created to add calories to MOM or DM without substantially increasing volume and without introducing a non-human milk-based nutritional product.
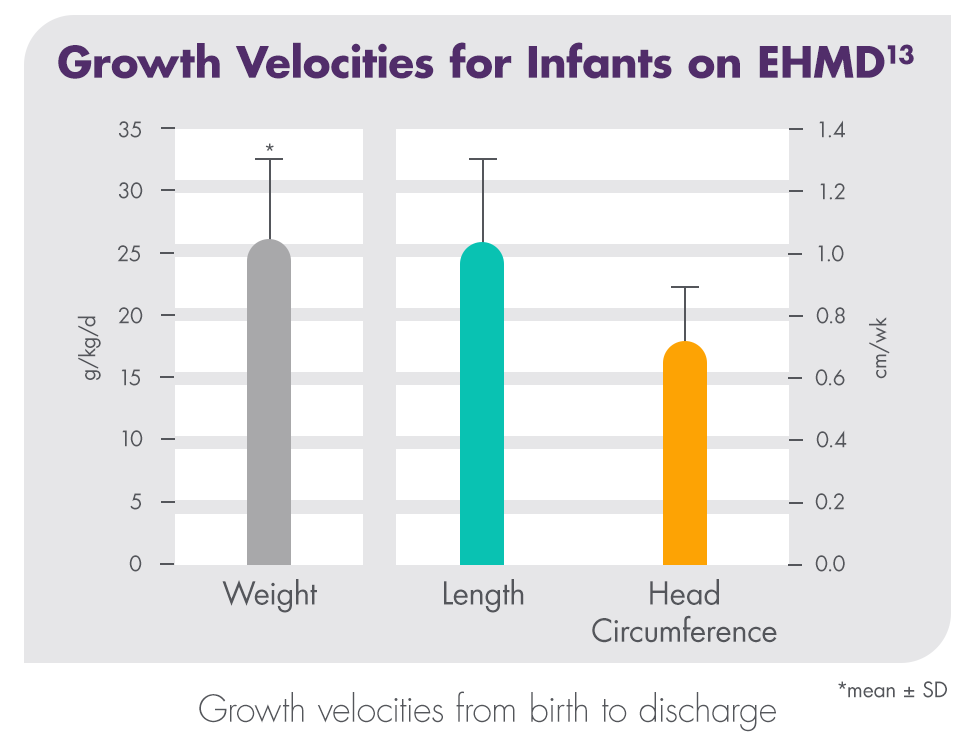
A randomized clinical trial found that premature infants who received an exclusive human milk diet (EHMD) with Prolact CR fortifier had superior length and weight velocity compared to infants who received an EHMD without Prolact CR fortifier.13
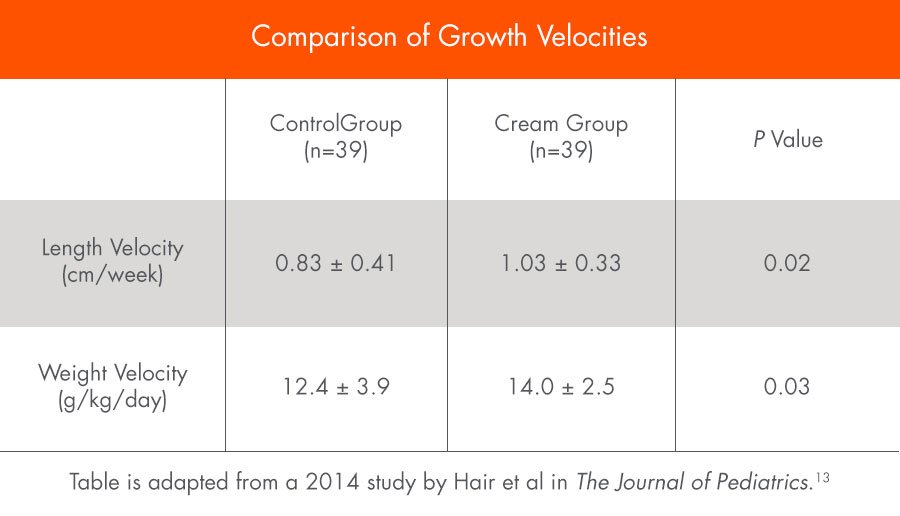
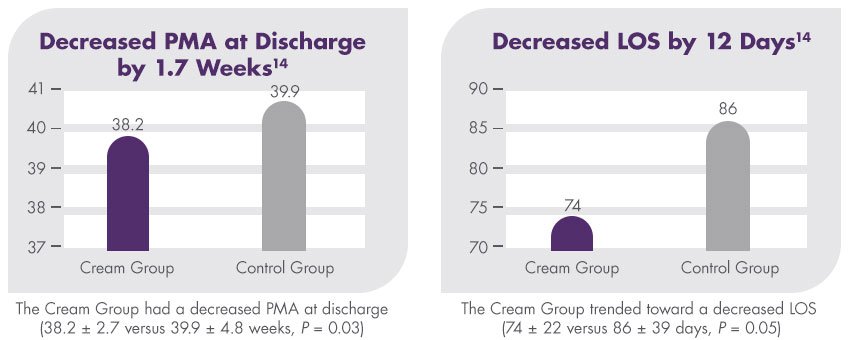
This study is a subset analysis of data originally published in 2014 by Hair et al in The Journal of Pediatrics.
Clinical Studies and Publications:
Prolact RTF (Ready-To-Feed) offers Neonatal Intensive Care Units superior solutions for their extremely premature infants when mother’s own milk is not available.
Human Milk-Based Premature Infant Formula

Pasteurized, standardized, quality human donor milk
The benefits of breastmilk are well established, and breastfeeding is highly recommended by healthcare professionals.
Since 2012, the American Academy of Pediatrics (AAP) has recommended the use of human milk for all preterm infants, whether MOM or pasteurized human donor milk, if MOM is unavailable.11
Guaranteed supply
Only Prolacta offers a guaranteed supply of donor human milk based on your NICU’s usage forecast – that means no more worrying about supply shortages.12

Prolact HM® Product Specification Sheet
Prolact HM® Supplemental Product Info
Prolact HM® Preparation Log Sheet
PremieLact Product Specification Sheet
Prolacta’s products, when used as part of an EHMD, have been shown to reduce the incidence of clinical complications.
In a multicenter, retrospective cohort study with 1587 patients, the outcomes of extremely premature infants (<1250 g birth weight) who received a diet including cow milk–based products were compared with infants who received an EHMD. The incidence of mortality, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), and late-onset sepsis were all reduced with an EHMD.13
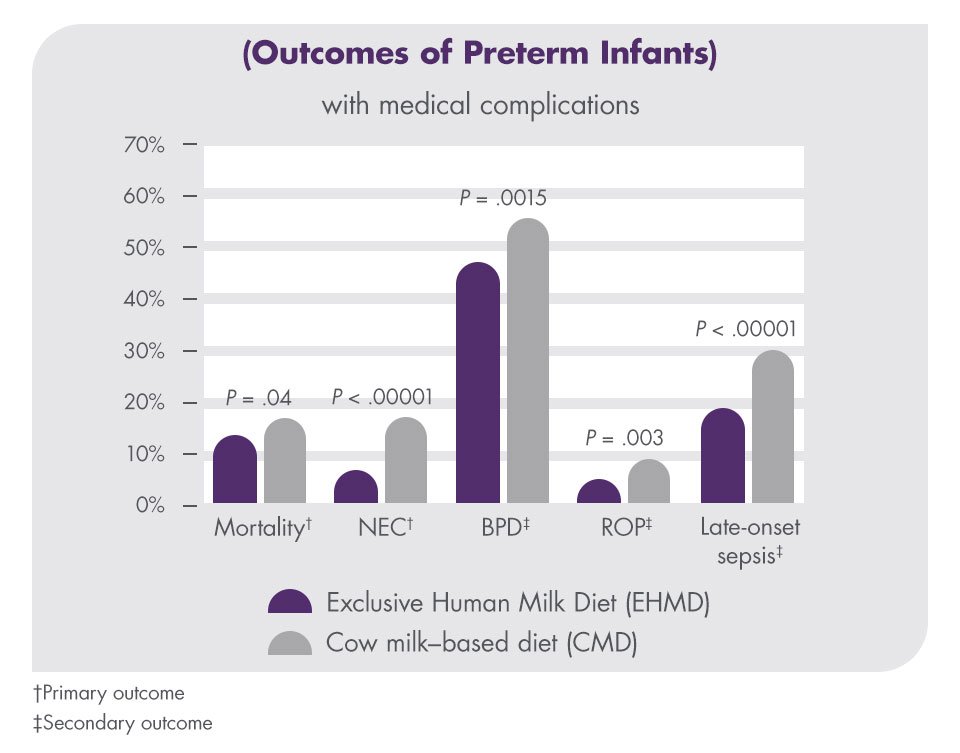
Very low birth weight babies are at risk for prematurity-related morbidities and interventions, such as BPD, ROP, late-onset sepsis, and NEC. The incremental cost of these morbidities and interventions can substantially increase the cost of NICU hospitalization.
Clinician-to-clinician guidance
Coding, billing, and reimbursement questions
Finding my sales representative
Support with an order